Cellulose-Based Stationary Phase Immobilized via Click Chemistry
October 10, 2025
On April 07, 2025, a groundbreaking study by Lehrhofer et al. published in Cellulose demonstrated the strategic application of Cu(I)-catalyzed azide-alkyne click chemistry to covalently immobilize a cellulose per(phenyl carbamate) chiral selector onto silica gel, overcoming critical limitations of traditional coated stationary phases. This research addresses the solvent-induced bleeding issue in HPLC enantiomer separation by developing a robust CSP (CSP1) through precise functionalization with propargyl anchor groups (DS=0.45) and efficient Huisgen cycloaddition onto azide-modified silica. The covalently fixed phase exhibited superior enantioselectivity, achieving baseline separation for diverse chiral analytes under normal-phase conditions, while enabling unprecedented solvent compatibility with strong eluents like THF and chloroform. Long-term stability tests confirmed minimal performance degradation after 33-hour flushing with chloroform-rich mobile phases, highlighting its potential for reliable, versatile method development in pharmaceutical and chemical analysis. This click chemistry approach marks a significant advancement in creating durable, high-performance chiral separation materials.
Introduction
In the fields of pharmaceutical development, agrochemicals, and fine chemicals, the separation of enantiomers—mirror-image molecules with potentially vastly different biological activities—is not just an analytical challenge but a critical necessity. High-performance liquid chromatography (HPLC) using chiral stationary phases (CSPs) is a powerful technique for this purpose. Among the most successful chiral selectors are derivatives of cellulose, a naturally chiral biopolymer. However, a significant limitation of traditional, physically coated CSPs is their susceptibility to solvent-induced damage, restricting the range of mobile phases that can be used for method optimization.
The Challenge: From Coated to Covalently Immobilized Phases
Traditional CSPs are made by coating a chiral selector, like cellulose tris(3,5-dichlorophenylcarbamate), onto porous silica gel. While highly effective under specific conditions, these coated materials have a critical weakness: the selector can dissolve or "bleed" from the column when exposed to strong organic solvents like tetrahydrofuran (THF) or chloroform. This bleeding degrades column performance, compromises reproducibility, and shortens the column's lifespan, limiting the chemist's toolbox for method development.
Covalent immobilization of the selector onto the silica support is the definitive answer to this problem. It unlocks the use of a much broader range of eluents, allowing for finer optimization of separations. The challenge, however, has been to develop an immobilization strategy that is both efficient and does not compromise the delicate, higher-order structure of the cellulose derivative, which is essential for its enantiorecognition capability.
The Click Chemistry Solution: A Strategic Synthesis
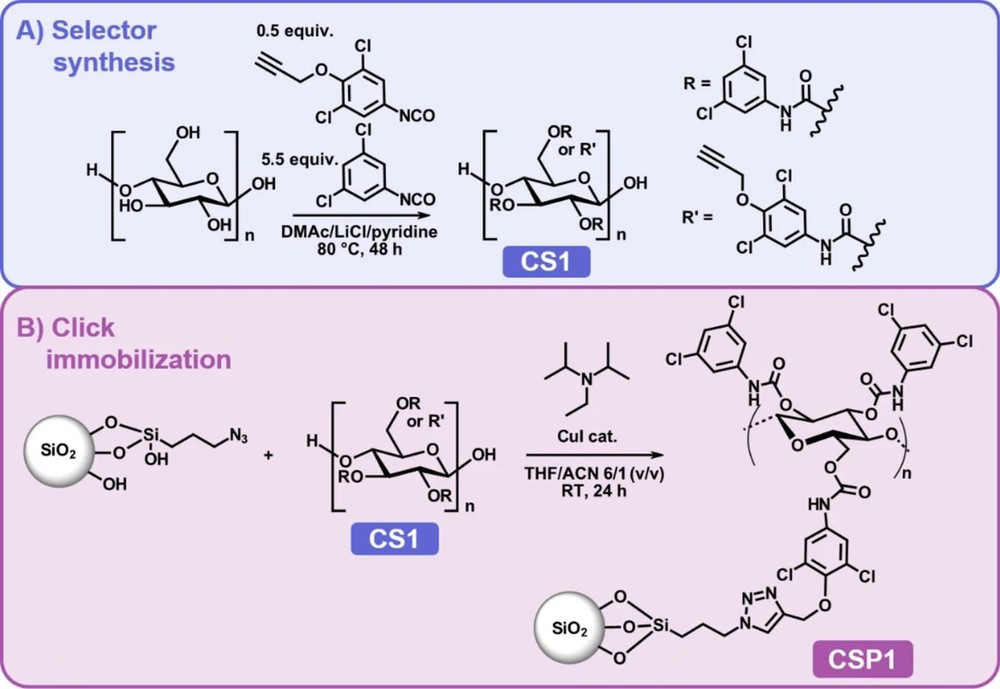
The research team designed a sophisticated chiral selector (CS1) based on cellulose. They functionalized it primarily with 3,5-dichlorophenyl carbamate groups for chiral recognition, but also introduced a small number of "anchor" groups: 4-propargyloxy-3,5-dichlorophenyl carbamate (with a degree of substitution, DS, of 0.45). This careful design ensures the majority of the selector's structure is dedicated to its enantioselective function, while the alkyne groups serve as specific handles for attachment.
The immobilization was performed on azide-functionalized silica gel (AzPS) via a Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition. This "click" reaction is renowned for its high efficiency, selectivity, and mild reaction conditions, which are crucial for preserving the integrity of the chiral selector. The result was a covalently bound CSP (CSP1) with a 9 wt.% selector loading. For comparison, coated phases with similar (CSP2, 9 wt.%) and higher (CSP3, 20 wt.%) loadings were also prepared.
 Fig.1 Synthesis and immobilization of cellulose-based chiral stationary phase CSP1. (Lehrhofer, et al., 2025)
Fig.1 Synthesis and immobilization of cellulose-based chiral stationary phase CSP1. (Lehrhofer, et al., 2025)
Superior Performance and Unmatched Stability
The performance of these phases was rigorously tested against a set of 16 diverse chiral analytes under standard normal-phase conditions (n-hexane/iso-propanol, 90:10).
- Enhanced Separation Power
The clicked phase CSP1 demonstrated superior performance compared to its coated counterpart CSP2 with the same nominal loading. It achieved baseline separation for several analytes where CSP2 failed. While the high-load coated phase CSP3 showed good resolution, it came with significantly longer retention times. CSP1 provided high-resolution separations with efficiency comparable to the lower-load coated phase, indicating that the covalent linkage, when done correctly, does not hinder but rather optimizes the selector's accessibility and structural arrangement.
- Unlocking Solvent Versatility
The most significant advantage of CSP1 was revealed in solvent compatibility tests. The researchers successfully used mobile phases containing high elution strength solvents like ethyl acetate, THF, and chloroform—conditions that would rapidly destroy a coated column. Remarkably, for some analytes, the addition of 5% of these solvents improved the resolution dramatically. For example, the resolution for one analyte increased from 3.95 to 6.47 with the addition of THF.
- Proven Long-Term Robustness
A stability test flushing CSP1 for 33 hours with a challenging mobile phase containing 20% chloroform showed no significant degradation in separation performance or retention times. This robust stability is a game-changer for laboratories requiring reliable, reproducible analyses over long periods and across diverse method developments.
Synergy with GlycoCLICK™ Expertise
The successful methodology described in this paper is a testament to the power of click chemistry in glycoscience and material functionalization. It aligns directly with the core expertise at GlycoCLICK, a brand of CD BioGlyco.
GlycoCLICK™-based Biomaterial Preparation Service
The creation of a functional HPLC column is a prime example of a high-value biomaterial. Our services extend to preparing sophisticated materials like nanoparticles, biosensors, and specialized scaffolds for applications in separation science, drug delivery, and diagnostics.
This research illustrates how click chemistry transforms a good chiral selector into an exceptional, durable analytical tool. The principles demonstrated—controlled functionalization, efficient conjugation, and enhanced material stability—are central to the solutions we provide.
Conclusion
The work by Lehrhofer et al. presents a significant advancement in chiral separation technology. By employing a controlled, click chemistry-based immobilization strategy, they have developed a cellulose-derived CSP that combines high enantioselectivity with exceptional solvent resistance and long-term stability. This approach opens new avenues for HPLC method development, making enantiomer separation more robust, versatile, and reliable.
For researchers looking to leverage these advanced techniques in their own projects—whether in analytical chemistry, drug discovery, or material science—GlycoCLICK offers the technology, expertise, and collaborative spirit to accelerate success. Explore our comprehensive suite of services to discover how our innovative GlycoCLICK platform can provide tailored solutions for your most challenging research needs.
Reference
- Lehrhofer, A.F., et al. Covalent anchoring of a cellulose per (phenyl carbamate) chiral selector onto silica gel through alkyne-azide click chemistry and its utilization in HPLC. Cellulose. 2025, 1-15.

 Fig.1 Synthesis and immobilization of cellulose-based chiral stationary phase CSP1. (Lehrhofer, et al., 2025)
Fig.1 Synthesis and immobilization of cellulose-based chiral stationary phase CSP1. (Lehrhofer, et al., 2025)